No more needles! Diabetics could soon have device that slowly inject insulin when needed
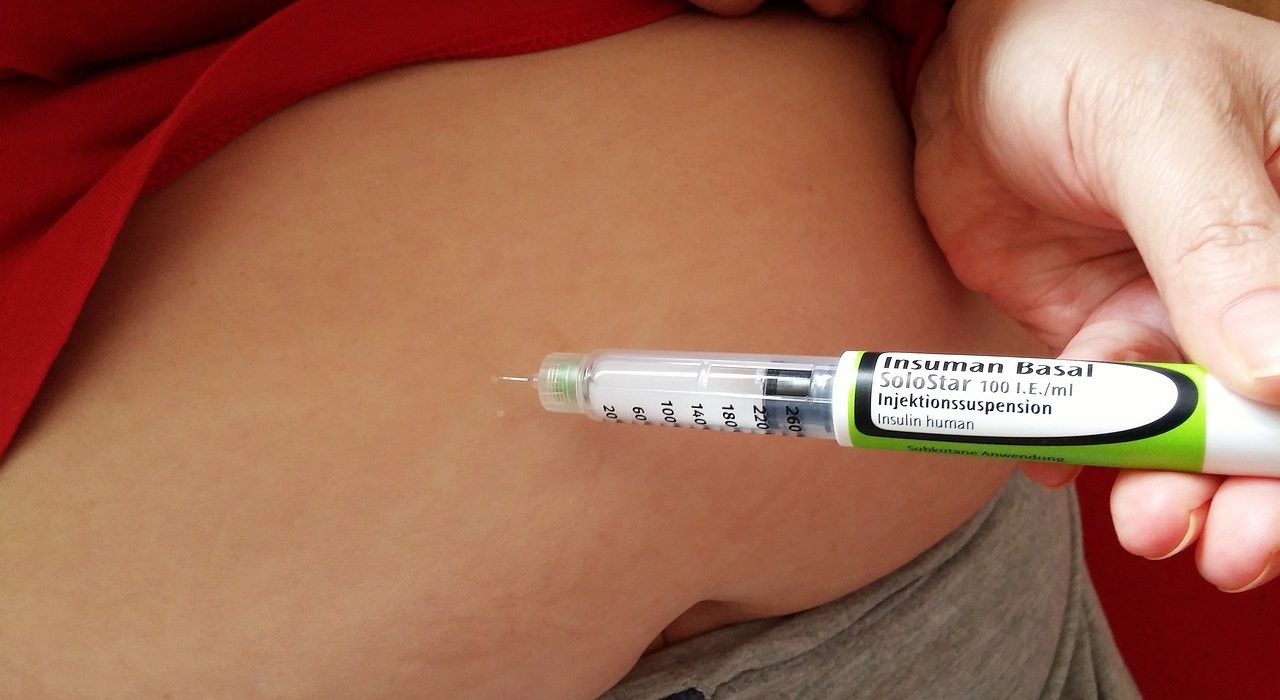
Scientists claim that a new implantable device, equipped with an ‘oxygen factory’, could soon replace insulin injections for individuals with type 1 diabetes. Researchers at the Massachusetts Institute of Technology have developed a device, similar in size to chewing gum, that generates a continuous supply of oxygen required to infuse a diabetic person’s body with vital insulin-producing cells. The device, which has been tested on mice, has the potential to eliminate the need for diabetics to constantly monitor their blood sugar levels and administer insulin injections. Furthermore, the device could be adapted to treat other diseases that require repeated protein deliveries.
Dr Daniel Anderson, a chemical engineering professor at MIT and a leader in the device’s development, describes it as a living medical device made from human cells that secrete insulin, along with an electronic life support system. The ability to manage type 1 diabetes without the tedious and painful processes of testing blood sugar and injecting insulin once a day would be a significant victory for the approximately two million Americans living with the condition. The device is expected to be tested on humans soon.
The daily routine of meticulously monitoring blood glucose levels and manually administering insulin is essential for the survival and well-being of individuals with diabetes. However, this process lacks the precise responsiveness of a non-diabetic body and fails to replicate the natural ability of the body to regulate blood sugar levels. According to Dr. Anderson, the majority of insulin-dependent diabetics who self-administer insulin struggle to maintain healthy blood sugar levels, even with careful attention. The limitations of this approach highlight the need for alternative solutions.
MIT scientists have developed a novel approach to address the challenge of supplying transplanted insulin-producing cells with sufficient oxygen to respond to blood glucose fluctuations. By splitting water vapor in the body into its component parts, hydrogen and oxygen, the system can deliver oxygen to the storage chamber that feeds transplanted insulin-producing cells. This enables the cells to respond immediately to surges in blood glucose levels, without the need for immunosuppressive drugs that can compromise the body’s immune system.
While some patients with diabetes have received transplanted cells from human corpses that can control diabetes, the need for immunosuppressive drugs to prevent rejection of the implanted cells remains a significant challenge. The innovative system developed by MIT researchers offers a promising alternative that could improve the lives of individuals with diabetes.
The scientists at MIT have developed a miniature device, comparable in size to a quarter, which was implanted beneath the skin of diabetic mice with fully functional immune systems. One group of mice received the implant with a water vapor-splitting membrane, while the other group received a device containing transplanted islet cells without any supplemental oxygen to maintain production of those cells. The mice that were given the implant maintained normal blood glucose levels relative to healthy animals, while the mice that received the device became hyperglycemic within approximately two weeks.
The small device operates without the need for wires or batteries and only requires a small voltage of approximately two volts generated through a phenomenon known as ‘resonant inductive coupling’. A tuned magnetic coil outside the body, which could be worn as a patch on the skin, transmits power to a small, flexible antenna within the device, allowing for wireless power transfer. Dr. Anderson expressed his team’s excitement about the progress the device has made, stating that “we really are optimistic that this technology could end up helping patients.”
Typically, when a medical device is implanted in the body, attacks by the immune system lead to a buildup of scar tissue called fibrosis, which can reduce the device’s effectiveness.
 Although scar tissue did form around the implants utilized in the study, the success of the device in regulating blood glucose levels indicates that insulin was still able to diffuse out of the device while glucose diffused into it. This innovative approach could also be employed to administer cells that produce other types of proteins required for extended periods of time. The MIT researchers demonstrated that their device could sustain cells that produce erythropoietin, a protein that stimulates the production of red blood cells.
Although scar tissue did form around the implants utilized in the study, the success of the device in regulating blood glucose levels indicates that insulin was still able to diffuse out of the device while glucose diffused into it. This innovative approach could also be employed to administer cells that produce other types of proteins required for extended periods of time. The MIT researchers demonstrated that their device could sustain cells that produce erythropoietin, a protein that stimulates the production of red blood cells.
Dr. Anderson expressed optimism that it will be feasible to create living medical devices that can reside in the body and produce drugs as necessary. Many diseases necessitate the exogenous administration of proteins, sometimes quite frequently. If a single implant could replace the need for biweekly infusions and function for an extended period, it could significantly benefit numerous patients. The researchers intend to test the device on larger animals and eventually on humans. Lead author on the study, MIT Research Scientist Siddharth Krishnan, stated that the materials employed are inherently stable and long-lasting, making long-term operation feasible, which is what they are currently working on.









